
INTRODUCTION
Thomson started the Cathode Ray experiment in the 1894 (19th century) at the Cavendish Laboratory at Cambridge University. He experimented with currents of electricity inside empty glass tubes.
THOMSON’S FIRST CATHODE RAY EXPERIMENT
Thomson’s first experiment was to investigate whether or not the negative charge could be separated from the cathode rays by magnetism.
To observe this he built a cathode ray tube with a metal cylinder on the end. This cylinder had two slits in it, leading to electrometers, which could measure small electric charges.
Thomson found that if the rays were magnetically bent and they could not enter the slit. Therefore, the electrometer registered little charge.
Thomson concluded that the negative charge was inseparable from the rays.
He found his conclusion by applying a magnetic field across the tube. Since the electrometers recorded no activity and so the charge had been bent away by the magnet.

THOMSON’S SECOND CATHODE RAY EXPERIMENT
Thomson’s second experiment was to prove that the rays carried a negative charge. His previous experiments did not prove this but he wanted to keep trying because he believed his previous experiment had been flawed because of the traced amounts of gas and a bad vacuum.
Thomson made a cathode ray with an almost perfect vacuum. This vacuum had two electrical pates halfway down the tube, one positively charged and one negatively charged. He knew that electrical charges repelled on another, which allowed him to see whether the charge was a positive or negative. He would observe this by looking at the light deflection pattern of the cathode ray
The rays were deflected by the electric charge. He concluded that cathode rays had a negative charge.
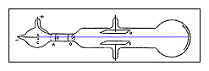
THOMSON’S THIRD EXPERIMENT-1896
Although Thomson had performed all these experiments he was still curious to know what was the size of the cathode rays.
Thomson used a charge-to-mass ratio because he knew the weight of the cathode ray tube, the heat, the electrical current, and how much heat had been added from the electrons firing.
He concluded that the negative cathode ray particles were a thousand times tinier than an atom.
Along these lines he proved the existence of subatomic particles.
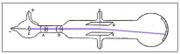
PROPOSAL
Since these puzzling rays of particles were a lot smaller than atoms Thomson concluded that they were microscopic pieces of atoms.
"He called these particles "corpuscles," and suggested that they might make up all of the matter in atoms. The “corpuscles” atoms were divisible."^1 (supposed to be a footnote- “J.J Thomson’s Cathode Ray Experiment.” Experiment-Resources.com. n.d. Web. 3 Oct. 2010.)
Thomson imagined the atom as being made up of these corpuscles swarming in an area of positive charge. This was his plum pudding model. Ernest Rutherford later proved this model incorrect.

IMPORTANCE
The significance of the mass-to-charge ratio is that two particles with the same mass-to-charge ratio travel in the same pathway in a vacuum when focused on the constant electric and magnetic fields.
LINK TO MY WORDLE
title="Wordle: Cathode Ray Experiment">
alt="Wordle: Cathode Ray Experiment"
style="padding:4px;border:1px solid #ddd">
CITATIONS
“J.J Thomson’s Cathode Ray Experiment.” Experiment-Resources.com. n.d. Web. 3 Oct. 2010. http://www.experiment-resources.com/cathode-ray.html#ixzz11WdqdUfL
Gibson, J.J. Newworldencylcopedia.. Web. 3 Oct. 2010.
http://www.newworldencyclopedia.org/entry/J._J._Thomson
“Cathode Ray Tube Experiments.” eHow.com. eHow, 22 January, 2010. Alexander Newman. Web. 3 Oct. 2010
http://www.ehow.com/list_6758342_cathode-ray-tube-experiments.html#ixzz11dE4AmEb
“The Experimental Discovery of Electron-Thomson Discover.” Think Quest. Oracle, n.d. Web. 3 Oct. 2010.
http://library.thinkquest.org/13394/angielsk/athompd.html
“3 Big Experiemnts, 1 Big Idea.” Aip. n.d. Web. 3 Oct. 2010
http://www.aip.org/history/electron/jj1897.htm
http://www.google.com/imgres?imgurl=http://www.wired.com/images/article/full/2008/04/jj_thompson_400px.jpg&imgrefurl=http://www.wired.com/science/discoveries/news/2008/04/dayintech_0430&usg=__IUmGDPHTcpIJSxWujx2XqqoSU74=&h=439&w=400&sz=66&hl=en&start=0&sig2=4_lAHKoiJPkWqXPXQ3gdlQ&zoom=1&tbnid=wq5V2qVyWsRpwM:&tbnh=136&tbnw=123&ei=VzatTOGsG4KKlweT1qXFBw&prev=/images%3Fq%3DJ.J%2BThomson%26um%3D1%26hl%3Den%26client%3Dfirefox-a%26rls%3Dorg.mozilla:en-US:official%26biw%3D1009%26bih%3D459%26tbs%3Disch:1&um=1&itbs=1&iact=rc&dur=312&oei=VzatTOGsG4KKlweT1qXFBw&esq=1&page=1&ndsp=12&ved=1t:429,r:2,s:0&tx=39&ty=115
http://www.google.com/imgres?imgurl=http://upload.wikimedia.org/wikipedia/commons/4/44/JJ_Thomson_Cathode_Ray_Tube_1.png&imgrefurl=http://www.ireference.ca/search/J.%2520J.%2520Thomson/&usg=__vlTqfx9MatmF-kSOiqib6lJbT70=&h=1144&w=916&sz=46&hl=en&start=0&sig2=h-M0Ssj35ndhKLn1TXO2EA&zoom=1&tbnid=GZr_iMcWGoY6gM:&tbnh=158&tbnw=127&ei=iDatTN6tBoLGlQejppjeBw&prev=/images%3Fq%3DJ.J%2BThomson%2Bfirst%2Bexperiment%26um%3D1%26hl%3Den%26client%3Dfirefox-a%26rls%3Dorg.mozilla:en-US:official%26biw%3D1009%26bih%3D459%26tbs%3Disch:1&um=1&itbs=1&iact=hc&vpx=767&vpy=115&dur=730&hovh=251&hovw=201&tx=182&ty=252&oei=iDatTN6tBoLGlQejppjeBw&esq=1&page=1&ndsp=8&ved=1t:429,r:7,s:0
http://www.google.com/imgres?imgurl=http://wikipremed.com/image_science_archive_th/020100_th/182300_JJ_Thomson_exp2_68.jpg&imgrefurl=http://www.wikipremed.com/image_archive.php%3Fcode%3D020100&usg=__1OrRxMZb1wG5KU_2wSALTiAVwIw=&h=150&w=200&sz=27&hl=en&start=8&sig2=Cb9wDj1zHCczkCVCAOEpKg&zoom=1&tbnid=qtYFBhAFpiMY9M:&tbnh=120&tbnw=160&ei=oTatTK_kE-fsnQfE7MHhDA&prev=/images%3Fq%3DJ.J%2BThomson%2Bfirst%2Bexperiment%26um%3D1%26hl%3Den%26client%3Dfirefox-a%26rls%3Dorg.mozilla:en-US:official%26biw%3D1009%26bih%3D459%26tbs%3Disch:10%2C399&um=1&itbs=1&iact=rc&dur=263&oei=iDatTN6tBoLGlQejppjeBw&esq=3&page=2&ndsp=10&ved=1t:429,r:5,s:8&tx=98&ty=65&biw=1009&bih=459
http://www.google.com/imgres?imgurl=http://reich-chemistry.wikispaces.com/file/view/plum.jpg/147037063/plum.jpg&imgrefurl=http://reich-chemistry.wikispaces.com/1875AD-1900AD&usg=___JthZ1ucFD-NWeEQAV-xr2lfx3s=&h=440&w=427&sz=39&hl=en&start=0&sig2=96EwbXYP6sgjgkSd_CfNtQ&zoom=1&tbnid=ETgwfgWz2i__2M:&tbnh=138&tbnw=129&ei=yDatTKzpBMqbnAeXn4yiCg&prev=/images%3Fq%3DJ.J%2BThomson%2Bplum%2Bpudding%2Bmodel%26um%3D1%26hl%3Den%26client%3Dfirefox-a%26rls%3Dorg.mozilla:en-US:official%26biw%3D1009%26bih%3D459%26tbs%3Disch:1&um=1&itbs=1&iact=hc&vpx=406&vpy=96&dur=336&hovh=228&hovw=221&tx=118&ty=126&oei=szatTLjROsSclgfWxNDCBw&esq=5&page=1&ndsp=10&ved=1t:429,r:2,s:0
http://www.google.com/imgres?imgurl=http://www.egglescliffe.org.uk/physics/particles/electron/jjexpt2.jpg&imgrefurl=http://www.egglescliffe.org.uk/physics/particles/electron/electron.html&usg=__AbqAuxh9bSz0ZX4M6LRMKJBsf4A=&h=71&w=209&sz=3&hl=en&start=0&sig2=j1q9X10Ho-7pxAkkH35Zfw&zoom=1&tbnid=4nd06LcZ3yT_oM:&tbnh=56&tbnw=167&ei=2zatTLTRFML7lwfE7u3DBw&prev=/images%3Fq%3DJ.J%2BThomsons%2Bsecond%2Bexperiment%26um%3D1%26hl%3Den%26client%3Dfirefox-a%26rls%3Dorg.mozilla:en-US:official%26biw%3D1009%26bih%3D459%26tbs%3Disch:1&um=1&itbs=1&iact=hc&vpx=176&vpy=194&dur=742&hovh=56&hovw=167&tx=135&ty=31&oei=2zatTLTRFML7lwfE7u3DBw&esq=1&page=1&ndsp=10&ved=1t:429,r:0,s:0